Pa Electronic Prescribing Of Controlled Substances
The Centers for Medicare Medicaid CMS has released a Request for Information RFI for Electronic Prescribing of Controlled Substances in Medicare Part D. All pharmacies across the state too must be capable of accepting those prescriptions and are required to immediately.

Don T Panic Over Mandated E Prescribing Of Controlled Substances Laws
780-101780-144 or substances that are controlled substances under Federal law.
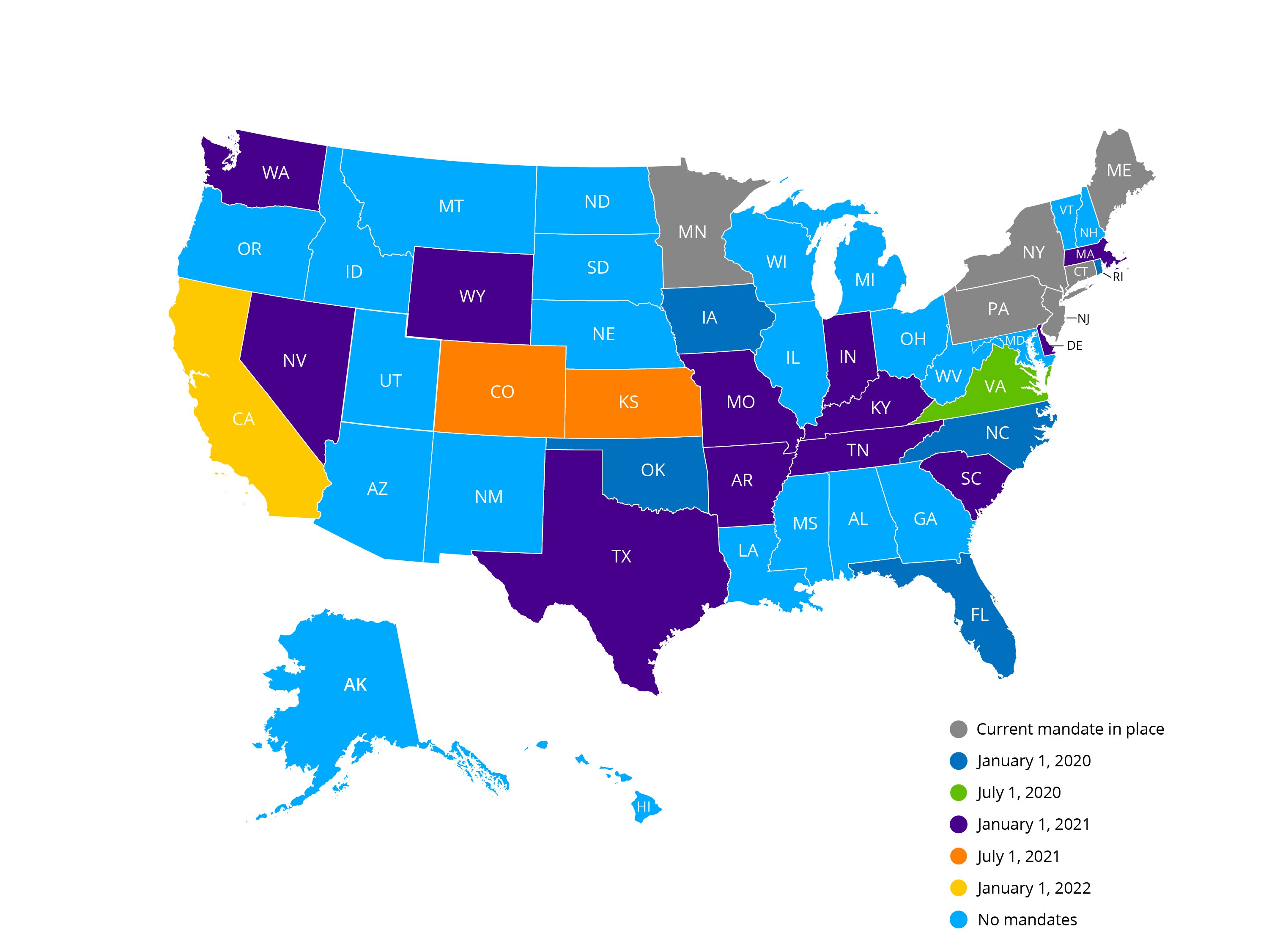
Pa electronic prescribing of controlled substances. Tom Wolf on Oct. The law Act 96 of 2018 mandates that all Schedule II through V controlled substances except when dispensed or administered directly to a patient by a practitioner. Eliminate dual workflows with Electronic Prescribing of Controlled Substances Reaching the tipping point for adoption of EPCS The volume of controlled substance prescriptions is on the rise increasing from 10 percent of all prescriptions in 2010 to a projected 30 percent in 2015.
The act requires health care practitioners with prescribing authority to prescribe schedule II III or IV controlled substances only via a prescription that is electronically transmitted to a pharmacy unless a specified exception applies. The Department of Health Department is currently in the process of promulgating EPCS regulations in accordance with the act. Per Act 96 of 2018 the Act prescribers excluding veterinarians are required to issue electronic prescriptions for Schedule II-V controlled substances as of October 24 2019 unless they meet an exception in the Act or in regulations.
Act 96- Electronic Prescribing of Controlled Substances in Pennsylvania begins October 24 Per Act 96 of 2018 practitioners excluding veterinarians will be required to issue electronic prescriptions for Schedule II-V controlled substances beginning Thursday October 24 2019. Effective January 1 2021 Texas Health and Safety Code 4810755 requires that prescriptions for controlled substances to be issued electronically except in limited circumstances or unless a waiver has been granted by the appropriate agency. Prescribing administering and dispensing.
Per Act 96 of 2018 the Act prescribers excluding veterinarians are required to issue electronic prescriptions for Schedule II-V controlled substances as of October 24 2019 unless they meet an exception in the Act or in regulations. This law replaces the traditional method of prescribing controlled substances. 1 2022 dentists and other prescribers in California must issue electronic-data prescriptions for both controlled and noncontrolled substances with very few exceptions.
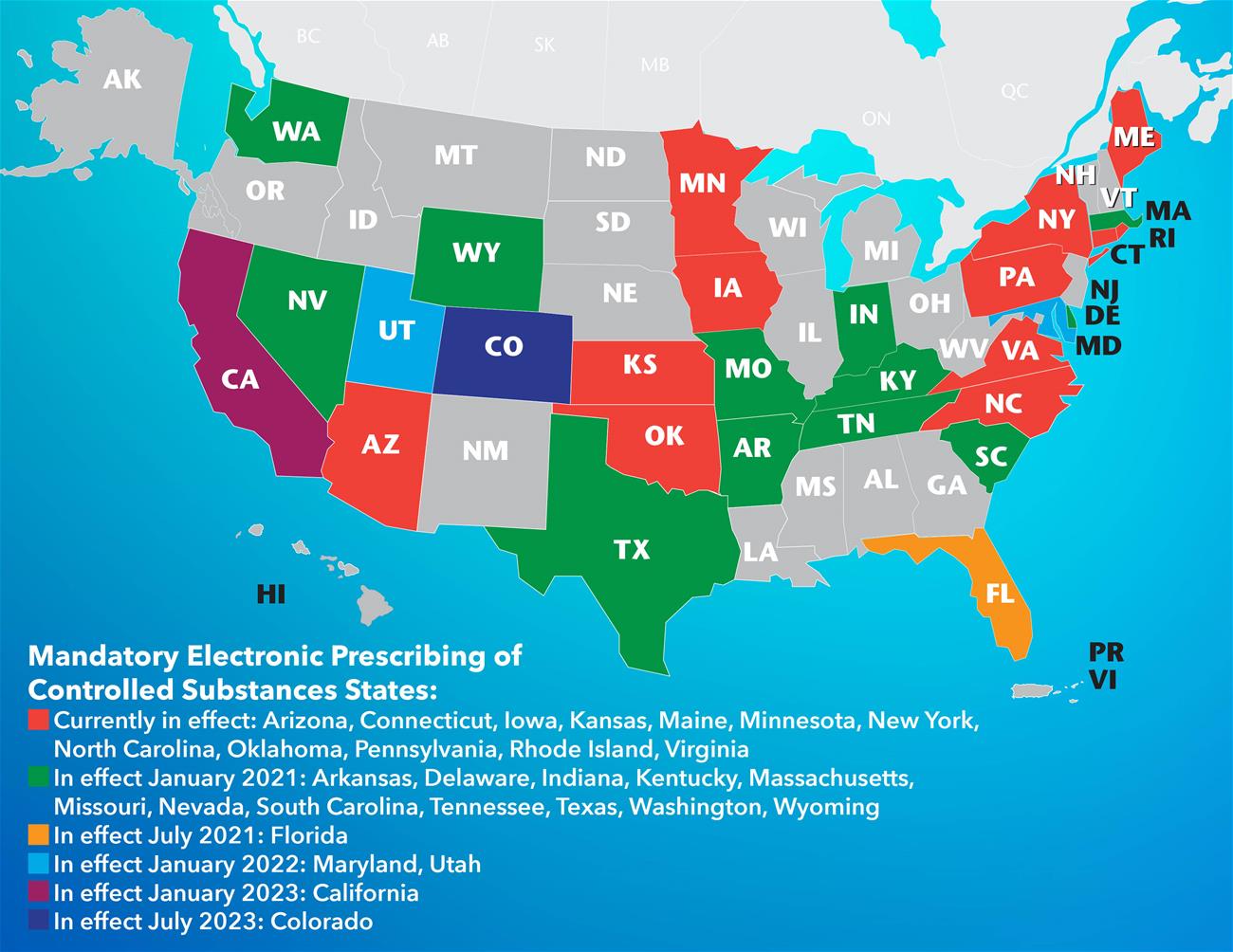
Electronic Prescribing of Controlled Substances Decreases Diversion Fraud. Electronic prescriptions of Schedule II controlled substances must comply with. Electronic prescribing of controlled substances EPCS is now required in Arizona for any prescription of a Schedule II controlled substance that is an opioid.
Harrisburg PA Governor Tom Wolf announced today that health care providers prescribing controlled substances are required to do so electronically unless they meet certain exceptions. This is set forth by Act 96 now in effect. Electronic prescribing for controlled substances.
Paper prescriptions will no longer be allowed by state law as CDA previously reported. Prescribing health care practitioners - electronic prescribing of controlled substances - exceptions. Electronic Prescribing of Controlled Substances among Medicare Part D Prescribers 2015-2016.
In 2010 the United States Drug Enforcement Administration DEA revised its regulations to allow pharmacies hospitals and health care providers to use electronic prescribing of controlled substances. Pennsylvania bill on electronic prescribing of controlled substances was signed into law by Gov. RCW 6950312 will require that prescriptions for Schedule II through V controlled substances or refill authorizations for a controlled substance included in Schedules III through V controlled substances be electronically communicated to pharmacies.
To increase EPCS in Arizona and prepare Arizona prescribers for the new state requirements mandated by the Arizona Opioid Epidemic Act Health Current has launched the 2019 EPCS Click for Control Campaign. Electronic prescribing of controlled substances EPCS has been permitted by the DEA since 20101 Beginning in 2015 all 50 states and the District of Columbia have approved EPCS for all schedules of drugs2 In 2017 77 of prescription medications were electronically prescribed3 However more than 90 of those electronically prescribed were for non-controlled dangerous drugs4 Only about 21 of. Sonal Parasrampuria MPH.
A major contributing factor to this increase is the reclassification of hydrocodone combination drugs Vicodin to. 1 Controlled substances under The Controlled Substance Drug Device and Cosmetic Act 35 P. The law Act 96 of 2018 mandates that all Schedule II through V controlled substances except when dispensed or administered directly to a patient by a practitioner or authorized agent other than a pharmacist to an ultimate user shall be prescribed electronically.
The RFI seeks input from stakeholders around implementation of Section 2003 of the Substance Use-Disorder Prevention that Promotes Opioid Recovery and Treatment for Patients and Communities Act SUPPORT Act which generally requires that prescriptions for controlled substances covered under a Medicare Part D prescription. The requirement to electronically prescribe starts on July 1 2021 for. Prescriptions for Schedule II controlled substances must be written with ink indelible pencil typewriter word processor computer printer or by electronic means and shall be manually signed by the prescriber except that prescriptions written by electronic means shall be electronically signed by the prescriber.
Pennsylvanias e-prescribing law took effect on Oct. The Department of Health Department is currently in the process of promulgating regulations in accordance with the Act. PA Electronic Prescription Requirement for Controlled Substances Takes Effect Beginning October 24 2019 pursuant to Act 96 of 2018 Act every licensed health care practitioner in Pennsylvania will be required to electronically prescribe controlled substances by sending the prescription directly to a pharmacy via the Internet.
Information on the E-prescribing requirements and wavier process is available here. Violation is subject to a civil penalty of 250 per violation up to 5000 per calendar year. A For purposes of this section drug includes the following.

Effectiveness Of Prescription Monitoring Programs In Reducing Opioid Prescribing Dispensing And Use Outcomes A Systematic Review The Journal Of Pain
Don T Panic Over Mandated E Prescribing Of Controlled Substances Laws
Https Www Health Pa Gov Topics Documents Programs Pa Epcs Faq Pdf

Epcs E Prescribe Controlled Substances Mdtoolbox E Prescribing

The Federal Controlled Substances Act A Primer For Providers

What Is Epcs Definition And Authentication Practice Fusion

Which States Require Epcs Newcroprx E Prescribing

Opioid Prescribing Safety Arizona Medical Association
Https Www Health Ny Gov Professionals Narcotic Electronic Prescribing Docs Epcs Faqs Pdf

Mdtoolbox Blog The Latest On E Prescribing Software And E Prescribing Integration

Which States Require Epcs Newcroprx E Prescribing
Mdtoolbox Blog The Latest On E Prescribing Software And E Prescribing Integration
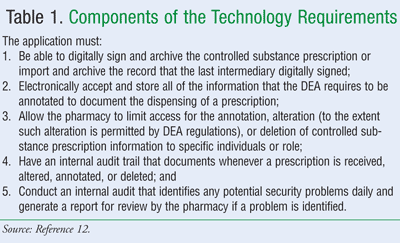
Electronic Controlled Substances Prescriptions

Florida Prescription Drug Database Pinellas Criminal Defense Attorney





Post a Comment for "Pa Electronic Prescribing Of Controlled Substances"